
Venor®GeMqEPutilizesquantitative,real-timePCR(qPCR).Thekitcanbeperformedwithanytypeofreal-timePCRcyclerabletodetectthefluorescencedyesFAM™andHEX™.Theprotocolprovidedispreferredforfastandreliablescreeningofcellculturesupernatantsmostapplicableinresearchanddevelopment.Thedetectionprocedurecanbeperformedwithin3hours.
MycoplasmasarespecificallydetectedbyamplifyingahighlyconservedrRNAoperon,ormorespecifically,the16SrRNAcodingregioninthemycoplasmagenome.Themycoplasma-specificamplificationisdetectedat520nm(FAM™channel).ThekitincludesprimerandFAM™labeledprobeswhichallowthespecificdetectionofallMollicutesspeciessofardescribedascontaminantsofcellculturesandmediacomponents.EukaryoticDNAisnotamplifiedbythisprimer/probesystem.
FalsenegativeresultsduetoPCRinhibitorsorimproperDNAextractionaredetectedbytheinternalamplificationcontrolwhichcanbeaddedtothePCRmastermix.Theamplificationoftheinternalamplificationcontrolisdetectedat610nm(HEX™channel).
TypeofPCR
TaqMan®based qPCRAssaywithFAM™andHEX™labeledprobes
RecommendedUse/Scope
Venor®GeMqEPisusedfordirectdetectionofMollicutes(Mycoplasma,Acholeplasma,Spiroplasma)contaminationincellculturesandcellmediacomponents.
KitComponents
lyophilizedprimer/nucleotide/probe/polymerase/mixinaliquotsof25tests
rehydrationbuffer
lyophilizedinternalamplificationcontrol
lyophilizedpositivecontrol
PCRgradewater
PackageSizes
- Cat.No.11-902525tests
Cat.No.11-9100100tests
Cat.No.11-9250250tests
ResultEvaluation
cyclerbased,real-timePCR
RequiredConsumables
PCRreactiontubes
OptionalforprocessvalidationandEP2.6.7complianttesting:
InternalControlDNAextra(4vialsfor300µleachofinternalamplificationcontrol;Cat.No.11-9905)
10CFU™SensitivityStandardsavailableforallEP 2.6.7listedmycoplasmaspecies
RequiredLabDevices
qPCRcyclerwithFAM™andHEX™filter
variablemicroliterPipettes
benchtopcentrifugefor1.5mlreactiontubes
ShelfLifeandStorage
Componentscanbestoredat+2to+8°Cforatleast12months.Afterrehydrationthereagentsmustbestoredat-18°C.
EP2.6.7Compliance
Yes.
Pleasenotethatvalidationdataareprovidedforinformationpurposeonly.EP2.6.7clearlystates“Wherecommercialkitsareused…,documentedvalidationpointsalreadycoveredbythekitmanufacturercanreplacevalidationbytheuser.Nevertheless,theperformanceofthekitwithrespecttoitsintendedusehastobedemonstratedbytheuser(e.g.detectionlimit,robustness,cross-detectionofotherclassesofbacteria.”.Pleasefeelfreetocontactusifyouneedfurtherassistance.
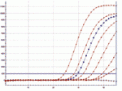
Fig.AmplifieddilutionseriesofMycoplasmafermentans,performedona
CorbettRotorGene®6000.



